Preserving cellular information
Understanding the role mutation rates play in aging and other traits
Introduction
DNA is a physical representation of the information required by an organism to live and reproduce. Does the rate at which that information is lost through mutation impact how long an organism can live?
There are layers to the information stored in the genome. Chemical changes to DNA—epigenetic modifications—encode additional information on top of the bases that they modify. DNA methylation is a crucial type of epigenetic modification that is lost over time. As a result, measuring methylation is one of the most reliable ways to predict biological age.1
But what about DNA? Does the rate at which information is lost through mutation impact the lifespan of an organism? An exciting recent study entitled “Somatic mutation rates scale with lifespan across mammals” sheds some light on this fundamental question. This preprint initially crossed my radar in August this year, when the first version was posted on bioRxiv. At that time, one of the lead authors Alex Cagan posted a Twitter thread providing a beautiful visual overview of the study.
Now, with a new draft of this work posted on December 14th, I want to distill down some of the insights from this study and share them with you here. This work was conducted by a talented team of researchers in the Martincorena Group2 at the Wellcome Sanger Institute. The lead authors of this study are Alex Cagan and Adrian Baez-Ortega.
Key Advances
It is essential for a germ cell (egg or sperm) to carry a copy of an organism’s genome in order to transmit this information to the progeny that it constructs. However, in astounding redundancy, somatic cells—the rest of the cells that comprise an organism’s body—each contain a copy of the genome. While this is a high school biology fact, it is worth reflecting on how remarkable this organizational feature is. This requires a tremendous amount of DNA replication and preservation.
A simple mental model of cell biology is that each cell in an organism contains an identical copy of the same genome, but reality is more complicated than this. Our somatic cells are accumulating mutations throughout our life, which means that we are more accurately seen as a mosaic of many slightly different genomes.
There are many fundamental questions to consider when thinking about somatic mutations. These mutations are the essential aberrations required by cancer cells. Are they also central to the process of aging? Does the rate at which these mutations occur vary across the tree of life—thereby explaining some of the wide variation in size and lifespan of organisms?
To address some of these questions, this study took a comparative approach. They compared somatic mutation rates across 16 different mammalian species in order to be able to see whether they correlated with traits such as lifespan. This included the naked mole-rat, a species widely studied for its atypical longevity and exceptionally low rates of cancer.3

This study used an interesting biological trick to measure these mutation rates. They sequenced cells collected from colorectal crypts, which are glands in the intestines of mammals that are known to originate in their development from a single initial stem cell. They are also easily visually detectable, making collection straightforward.4
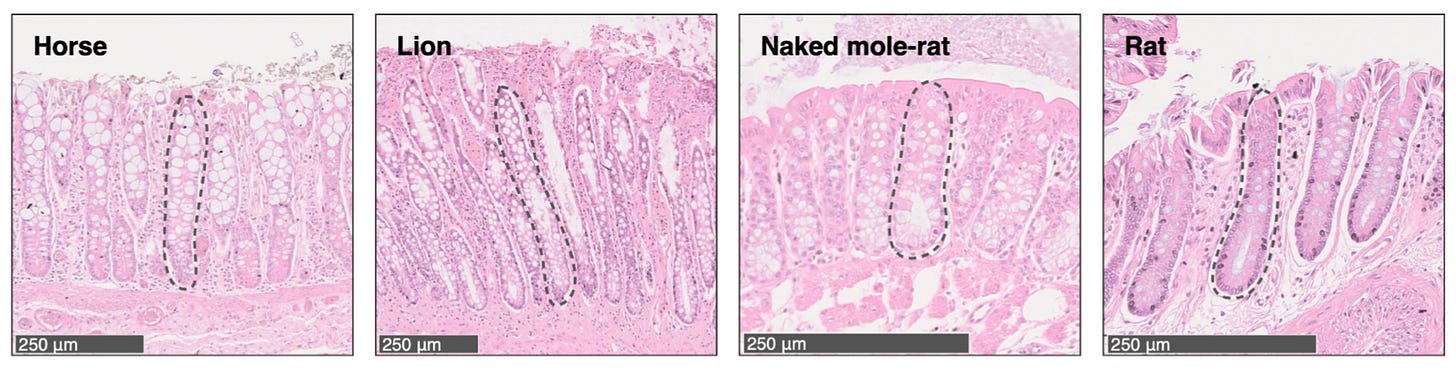
With mutation rates measured in a wide range of mammals, they were able to begin to address some of the fundamental questions about somatic evolution.
Results
As I’ve mentioned, patterns of DNA methylation are a robust signal for predicting biological age. This could be interpreted as a loss of important regulatory information over time, which contributes to the process of aging. In a similar fashion, there is the somatic mutation theory of aging, which proposes that the accumulation of mutations is an important driver of aging. This can be viewed in information theoretic terms: entropy increases within living systems as cellular information is lost through mutation.
This study found evidence confirming a long-standing prediction of this theory: there is an inverse relationship between somatic mutation rates and lifespan.
This is compelling empirical evidence that somatic mutations may play an important role in the aging process.5 They may serve as both the engine for cancer formation and biological decay. Our lifespan may be contingent on our evolved cellular machinery that continually repairs and protects the information stored in DNA. Our cells are locked in a constant battle against entropy.
Another central question surrounding somatic mutation rates is how they relate to the size of an organism. There is a strange observation called Peto's Paradox which is that contrary to naive expectation, the incidence of cancer does not correlate with the number of cells in an organism. Probabilistically, if all cells have some likelihood of becoming cancerous, more cells should translate into a higher likelihood of cancer overall for an organism. But some enormous species such as whales actually have a lower incidence of cancer than humans. Could larger species have evolved to have a lower rate of somatic mutations?
As can be seen above, this study didn’t find particularly strong evidence that this is the case. This is actually consistent with existing observations that show other possible mechanistic explanations for the low incidence of cancer in larger species. One cool example of this type of evidence is that elephants have more copies of TP53, which is a crucially important gene for tumor suppression. Having more copies could provide redundant protection against its function being lost through mutation, providing an alternative model for resolving Peto’s Paradox that is consistent with the weak relationship between mutation rates and body mass seen in this study.6
The authors of this study also drilled down into their sequencing data in order to compare the underlying mutational signatures across species, finding interesting commonalities. These results are worth checking out in the full preprint.
Final Thoughts
One core aspect of living systems is that they use energy to maintain internal order in the midst of a chaotic world constantly becoming more disordered. For life on Earth, maintaining the information physically represented in DNA is of crucial importance for cells and organisms. Up until now, it hasn’t been clear how the rate at which mutations occur—and information is lost—varies between organisms. Do longer lived organisms manage to accumulate less mutations over time?
This study has generated empirical data that begins to address questions about how mutation rates vary between different mammals. Through a clever comparative analysis, we now have evidence that supports one of the main hypotheses of the somatic mutation theory of aging. This work also provides more evidence that the answer to Peto’s paradox lies outside somatic mutation rates.
Thanks for reading this highlight of “Somatic mutation rates scale with lifespan across mammals” from the Martincorena Group. If you’ve enjoyed this post and don’t want to miss the next one, you can sign up to have them automatically delivered to your inbox:
Until next time! 🧬
If you’re interested in this type of prediction, I wrote a post about a new sequencing technology that can make these measurements in a very cost-effective way.
This group has produced some of the best work on this topic in the world, including a new sequencing technology for better detecting mutations present within individual DNA molecules. If the work highlighted here captures your interest, considering exploring more of the work from this lab.
There is a large amount to study in this organism including their highly unusual metabolism, extraordinarily high translational fidelity, and extreme protein stability, and several “anti-cancer” genes.
They isolated the crypt cells for sequencing from histology slides using a technique called laser-capture microdissection (LCM). This is another reason I enjoyed this paper. It reminds me of a project I was involved in while at the CVI where we did LCM and RNA-seq. The core facility with the instrument was buried in the labyrinthine Seattle Veteran’s Hospital, making for a memorable adventure.
As good scientists, the authors highlighted other possible explanations of this observation. For example, cell division rates also increase with age, potentially explaining part of this. Another is that this is an artifact of selective pressure on to reduce the rate of mutations in the germ line. For a more in depth breakdown, check out the full preprint.
Will our future descendants walk around with TP53 copy number expansions and rates of cancer incidence as low as elephants?



