Plant genetic circuits
Engineering root structure in plants using synthetic biology
Biology and engineering are coming together in profound ways. The potential is for civilization-scale flourishing, a world of abundance not scarcity, supporting a growing global population without destroying the planet.
- Drew Endy
Introduction
At times, it can be easy to conflate biotechnology with the development of new therapeutics. As biological beings ourselves, we tend to myopically focus on using our growing capacity to engineer biology to improve our health. We want to live longer, mitigate disease, defeat cancer. While these are noble pursuits, they only scratch the surface of what biological technology could accomplish.
In the post-industrial world, we often forget that biology is the ultimate distributed manufacturing platform. Just think of the incredible amount of mass continually created across all corners of our globe by biological life. The generative capacity of the global ecosystem is far greater than that of human industry. While biotechnology holds the promise of improving and extending human health, it also offers the potential of tapping into this profound manufacturing capacity for an abundant future.
At our current stage, we can only begin to imagine what might be possible if we could fully harness living systems as an engineering substrate. What if we could grow our homes instead of build them? We have already used recombinant DNA technology to spread vaccine manufacturing capacity across the world. As biotechnology matures, what else could this general pattern be applied to? Could we design new food, clothing, and materials, share the genetic instructions with each other over the Internet, and grow the items locally?
Of course, the only path forward to realize any of these aspirations is to expand our ability to design and engineer biology. This is what synthetic biology aims to accomplish. Historically, synthetic biology has heavily focused on using well understood microbes such as E. coli as the “chassis” for engineering new biological processes—which at times has been criticized as a shortcoming of the field. Leaders in synthetic biology have emphasized the need to move beyond microbes, and to expand the ability to engineer mammalian cells.
But what about plants? Plants grow to an astounding number of different sizes and shapes, feed us, clothe us, provide the majority of our materials, and are estimated to constitute ~80% of the total biomass on Earth. Despite their central role in our existence, plants have been largely ignored in synthetic biology because of the difficulty associated with engineering them.
This is beginning to change—the field of plant synthetic biology is gaining traction. I was very excited to see a new preprint entitled “Synthetic genetic circuits enable reprogramming of plant roots” which was a beautiful example of work in this space. This work explores how to control plant root branch density using logical circuits implemented with DNA.
This work was led by Jennifer Brophy while she was a postdoc in the Dinneny Lab at Stanford. She recently started her own lab at Stanford, so aspiring plant synthetic biologists should definitely reach out!
Key Advances
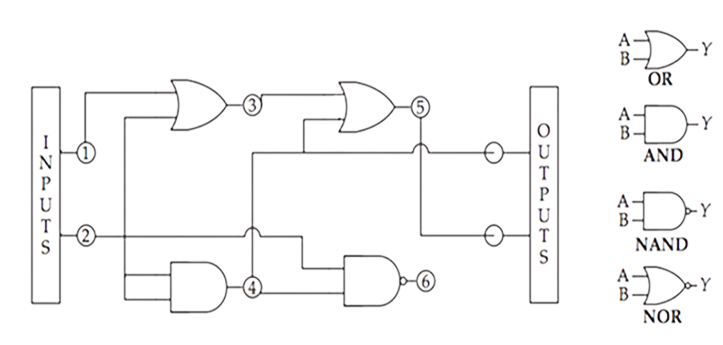
What is a genetic circuit? It can be useful to start by describing what a digital circuit is.
A digital circuit is a system that takes a set of binary inputs and computes a predictable output by performing logical operations. Cells have been shown to be capable of circuit-like behavior such as the lac operon, where E. coli can switch up their metabolic machinery to consume lactose when they sense an absence of glucose. This type of genetic system that computes on inputs to change biological outputs is a genetic circuit.

A central goal of synthetic biology has been to improve our ability to design new genetic circuits capable of carrying out complex processes. As I like to emphasize on occasion, this type of computation is not a metaphor. An active area of research has been to establish a hardware description language for cells, where engineers can describe their intended process, and compile it into a DNA sequence with the appropriate circuits.
As I’ve mentioned, most of this work has been done in microbes or mammalian cells. Developing plant genetic circuits has suffered technical challenges. To start, the time scale for actually transforming plants with DNA is much longer than for microbes because of the considerable difference in the length of their life cycle. It’s also more difficult to work with a multicellular organism that has different cell types.
One of the major insights of this work is that they used a quantitative transient expression assay to experimentally tune their genetic circuits. This type of assay measures the temporary change in expression directly after novel DNA has been introduced—offering much faster feedback than a stable expression assay which relies on the DNA actually being integrated. This approach made circuit development experimentally feasible.
Results
The overarching goal of this study was to design genetic circuits in plants, and then use them to control root density.

They were specifically interested in lateral root density, which quantifies the number of outgrowths branching to the side from the main descending root. A higher lateral root density enables plants to better sample their environment for water and nutrients. Being able to precisely engineer this type of trait has global implications—this type of technology may be essential for sustaining agriculture in the future, and could be used as a tool to mitigate challenges plants will face in a warming climate.
With this high-level goal in mind, the researchers did what good engineers do: they decomposed their larger problem into a smaller set of problems that could be tackled one at a time. To control root density, they needed circuits. In order to engineer circuits, they needed individual regulators of gene expression. They engineered a set of transcriptional activators and repressors, and tested how well they could modulate expression in Nicotiana benthamiana.
I think that this is a great figure for several reasons. To start, they had real success with transcriptional activation. You can observe from the ratios of expression between the control and the activated plants that 9 out of 10 of the activators worked. Equally important is the fact that 4 out of 10 of the repressors worked—and that it was clearly communicated in the first figure of the paper. Physical reality is an unforgiving substrate. Humility and intellectual honesty are both required to make progress with this type of engineering.
They proceeded to tune and improve the designs of their transcriptional regulators, seeing gains in performance for both activators and repressors. With their building blocks measured and optimized, they moved towards composing circuits.
As can be seen above, they tested circuits where expression was a function of logical operations performed on the individual transcriptional regulators. They could drive expression with one input (A and B), or carry out more complex logic like the NOT IMPLIES (NIMPLY) gates. Take for instance, A NIMPLY B, where expression takes place without either input, with just B, or with A and B combined, but is absent for just A alone.
Finally, with tuned circuits in hand, they turned their attention to engineering root density in Arabidopsis.1 They set out to modulate the expression of the solitary root (slr-1) gene which eliminates root branching—resulting in lower lateral root density. A key issue with engineering this gene is that it has pleiotropic effects—meaning that it impacts several different traits. With their circuits, they were able to change its expression only in a specific cell type, circumventing this problem.
As can be seen above, they were able to quantitatively control root density based on the amount of slr-1 expression that they drove with their logic gate. Because of the specificity of the gate to a single cell type, none of the other traits controlled by slr-1 were impacted.
Final Thoughts
Plants are one of the greatest examples of the diversity of form and function that biology is capable of generating. As we expand our capacity to engineer biology, it is impossible to imagine all that might be possible to accomplish in partnership with plants. Plant synthetic biology could become a critical discipline for feeding and clothing a growing global population on a warming planet. It may also be an essential technology as we dream of a multiplanetary future. Realizing any of these dreams will require a considerable amount of effort and engineering—so let’s get to it!
Thanks for reading this highlight of “Synthetic genetic circuits enable reprogramming of plant roots.” If you’ve enjoyed this post and don’t want to miss the next one, you can sign up to have them automatically delivered to your inbox:
Until next time! 🧬
While the regulators and circuits were originally developed in Nicotiana benthamiana, they were able to successfully transfer them for use in Arabidopsis thaliana, which is one of the most widely studied plant model organisms.